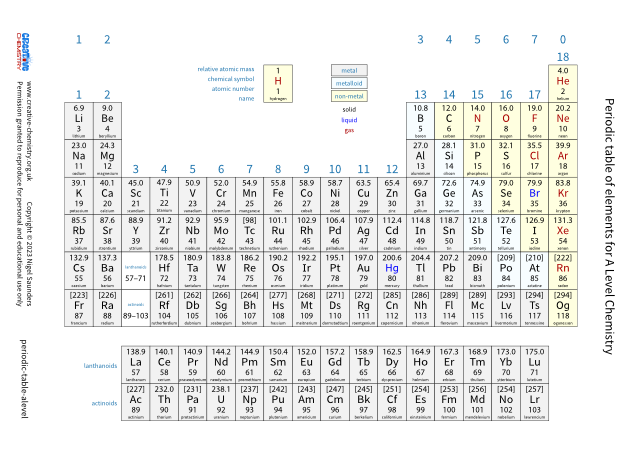
Periodic table (A Level)
What is here?
The periodic table below is based on the ones used by the different examination boards.
The group numbers 1 to 18 were recommended by IUPAC in 1988. They supersede the older numbering system (groups 1 – 0, or 1 – 8) but the older system is still in common use.
There is a summary at the bottom of the page. It shows the main differences between this table and the ones used by three examination boards.
Select an element using your mouse (or finger on touch devices). You will see its melting and boiling points, electronic configuration (first 36 elements only), and if it is radioactive or toxic.
Hydrogen
Melting point: –259.2 °C
Boiling point: –252.9 °C
1s1
Helium
Melting point: –272.2 °C
Boiling point: –268.9 °C
1s2
Lithium
Melting point: 180.5 °C
Boiling point: 1330 °C
1s2 2s1
Beryllium
Melting point: 1287 °C
Boiling point: 2469 °C
1s2 2s2
Boron ![]()
Melting point: 2076 °C
Boiling point: 3927 °C
1s2 2s2 2p1
Carbon
Sublimes at 3642 °C
solid → gas
1s2 2s2 2p2
Nitrogen
Melting point: –210 °C
Boiling point: –195.8 °C
1s2 2s2 2p3
Oxygen
Melting point: –218.8 °C
Boiling point: –183 °C
1s2 2s2 2p4
Fluorine ![]()
Melting point: –219.7 °C
Boiling point: –188.1 °C
1s2 2s2 2p5
Neon
Melting point: –248.6 °C
Boiling point: –246.1 °C
1s2 2s2 2p6
Sodium
Melting point: 97.8 °C
Boiling point: 882.9 °C
1s2 2s2 2p6 3s1
Magnesium
Melting point: 650 °C
Boiling point: 1091 °C
1s2 2s2 2p6 3s2
Aluminium
Melting point: 660.3 °C
Boiling point: 2470 °C
1s2 2s2 2p6 3s2 3p1
Silicon
Melting point: 1414 °C
Boiling point: 3265 °C
1s2 2s2 2p6 3s2 3p2
Phosphorus
Melting point: 44.2 °C
Boiling point: 280.5 °C
1s2 2s2 2p6 3s2 3p3
Sulfur
Melting point: 115.2 °C
Boiling point: 444.6 °C
1s2 2s2 2p6 3s2 3p4
Chlorine
Melting point: –101.5 °C
Boiling point: –34.0 °C
1s2 2s2 2p6 3s2 3p5
Argon
Melting point: –189.3 °C
Boiling point: –185.9 °C
1s2 2s2 2p6 3s2 3p6
Potassium
Melting point: 63.5 °C
Boiling point: 759 °C
1s2 2s2 2p6 3s2 3p6 4s1
Calcium
Melting point: 842 °C
Boiling point: 1484 °C
1s2 2s2 2p6 3s2 3p6 4s2
Scandium
Melting point: 1541 °C
Boiling point: 2836 °C
1s2 2s2 2p6 3s2 3p6 4s2 3d1
Titanium
Melting point: 1668 °C
Boiling point: 3287 °C
1s2 2s2 2p6 3s2 3p6 4s2 3d2

Vanadium
Melting point: 1910 °C
Boiling point: 3407 °C
1s2 2s2 2p6 3s2 3p6 4s2 3d3
Chromium ![]()
melting point: 1907 °C
Boiling point: 2671 °C
1s2 2s2 2p6 3s2 3p6 4s1 3d5
Manganese
Melting point: 1246 °C
Boiling point: 2061 °C
1s2 2s2 2p6 3s2 3p6 4s2 3d5
Iron
Melting point: 1538 °C
Boiling point: 2862 °C
1s2 2s2 2p6 3s2 3p6 4s2 3d6

Cobalt
Melting point: 1495 °C
Boiling point: 2927 °C
1s2 2s2 2p6 3s2 3p6 4s2 3d7
Nickel
Melting point: 1455 °C
Boiling point: 2730 °C
1s2 2s2 2p6 3s2 3p6 4s2 3d8
Copper
Melting point: 1085 °C
Boiling point: 2562 °C
1s2 2s2 2p6 3s2 3p6 4s1 3d10
Zinc
Melting point: 419.5 °C
Boiling point: 907.0 °C
1s2 2s2 2p6 3s2 3p6 4s2 3d10

Gallium
Melting point: 29.76 °C
Boiling point: 2400 °C
1s2 2s2 2p6 3s2 3p1 4s2 3d10 4p1
Germanium
Melting point: 938.3 °C
Boiling point: 2833 °C
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
Arsenic ![]()
Sublimes at 615 °C
solid → gas
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3
Selenium ![]()
Melting point: 221 °C
Boiling point: 685 °C
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
Bromine
Melting point: –7.2 °C
Boiling point: 58.8 °C
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
Krypton
Melting point: –157.4 °C
Boiling point: –153.4 °C
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Rubidium
Melting point: 39.3 °C
Boiling point: 688 °C
Strontium ![]()
Melting point: 777 °C
Boiling point: 1377 °C
Yttrium
Melting point: 1526 °C
Boiling point: 2930 °C
Zirconium
Melting point: 1855 °C
Boiling point: 4377 °C
Niobium
Melting point: 2477 °C
Boiling point: 4744 °C
Molybdenum
Melting point: 2623 °C
Boiling point: 4639 °C
Technetium ![]()
Melting point: 2157 °C
Boiling point: 4265 °C
Ruthenium
Melting point: 2334 °C
Boiling point: 4150 °C
Rhodium ![]()
Melting point: 1964 °C
Boiling point: 3695 °C
Palladium
Melting point: 1555 °C
Boiling point: 2963 °C
Silver
Melting point: 961.8 °C
Boiling point: 2162 °C
Cadmium ![]()
Melting point: 321 °C
Boiling point: 767 °C
Indium
Melting point: 156.6 °C
Boiling point: 2072 °C
Tin
Melting point: 231.9 °C
Boiling point: 2602 °C
Antimony
Melting point: 630.6 °C
Boiling point: 1635 °C
Tellurium ![]()
Melting point: 449.5 °C
Boiling point: 988 °C
Iodine
Melting point: 113.7 °C
Boiling point: 184.3 °C
Xenon
Melting point: –111.7 °C
Boiling point: –108.1 °C
Caesium
Melting point: 28.5 °C
Boiling point: 671 °C
Barium ![]()
Melting point: 727 °C
Boiling point: 1845 °C
Lanthanum
Melting point: 920 °C
Boiling point: 3464 °C
Cerium
Melting point: 795 °C
Boiling point: 3443 °C
Praseodymium
Melting point: 935 °C
Boiling point: 3130 °C
Neodymium
Melting point: 1024 °C
Boiling point: 3074 °C
Promethium ![]()
Melting point: 1042 °C
Boiling point: 3000 °C
Samarium
Melting point: 1072 °C
Boiling point: 1900 °C
Europium
Melting point: 826 °C
Boiling point: 1529 °C
Gadolinium ![]()
Melting point: 1312 °C
Boiling point: 3000 °C
Terbium
Melting point: 1356 °C
Boiling point: 3123 °C
Dysprosium
Melting point: 1407 °C
Boiling point: 2562 °C
Holmium
Melting point: 1461 °C
Boiling point: 2600 °C
Erbium
Melting point: 1529 °C
Boiling point: 2868 °C
Thulium
Melting point: 1545 °C
Boiling point: 2223 °C
Ytterbium
Melting point: 824 °C
Boiling point: 1196 °C
Lutetium
Melting point: 1652 °C
Boiling point: 3402 °C
Hafnium
Melting point: 2233 °C
Boiling point: 4603 °C
Tantalum
Melting point: 3017 °C
Boiling point: 5458 °C
Tungsten
Melting point: 3422 °C
Boiling point: 5930 °C
Rhenium
Melting point: 3186 °C
Boiling point: 5630 °C
Osmium
Melting point: 3033 °C
Boiling point: 5012 °C
Iridium
Melting point: 2446 °C
Boiling point: 4130 °C
Platinum
Melting point: 1768 °C
Boiling point: 3825 °C
Gold
Melting point: 1064 °C
Boiling point: 2970 °C

Mercury ![]()
Melting point: –38.8 °C
Boiling point: 356.7 °C

Thallium ![]()
Melting point: 304 °C
Boiling point: 1473 °C
Lead ![]()
Melting point: 327.5 °C
Boiling point: 1749 °C
Bismuth
Melting point: 271.5 °C
Boiling point: 1564 °C
Polonium ![]()
Melting point: 254 °C
Boiling point: 962 °C
Astatine ![]()
Melting point: 302 °C
Boiling point: 337 °C
Radon ![]()
Melting point: –71 °C
Boiling point: –61.7 °C
Francium ![]()
Melting point: 27 °C
Boiling point: 677 °C
Radium ![]()
Melting point: 700 °C
Boiling point: 1737 °C
Actinium ![]()
Melting point: 1050 °C
Boiling point: 3200 °C
Thorium ![]()
Melting point: 1750 °C
Boiling point: 4788 °C
Protactinium ![]()
Melting point: 1568 °C
Boiling point: 4027 °C
Uranium ![]()
Melting point: 1132 °C
Boiling point: 4131 °C
Neptunium ![]()
Melting point: 639 °C
Boiling point: 4174 °C
Plutonium ![]()
Melting point: 639 °C
Boiling point: 3228 °C
Americium ![]()
Melting point: 1176 °C
Boiling point: 2607 °C
Curium ![]()
Melting point: 1340 °C
Boiling point: 3110 °C
Berkelium ![]()
Melting point: 986 °C
Bp (estimated): 2627 °C
Californium ![]()
Melting point: 900 °C
Bp (estimated): 1470 °C
Einsteinium ![]()
Melting point: 860 °C
Bp (estimated): 996 °C
Fermium ![]()
Mp (predicted): 1855 °C
Boiling point: unknown
Mendelevium ![]()
Mp (predicted): 827 °C
Boiling point: unknown
Nobelium ![]()
Mp (predicted): 827 °C
Boiling point: unknown
Lawrencium ![]()
Mp (predicted): 1627 °C
Boiling point: unknown
Rutherfordium ![]()
Mp (predicted): 2100 °C
Bp (predicted): 5500 °C
Dubdium ![]()
Melting point: unknown
Boiling point: unknown
Seaborgium ![]()
Melting point: unknown
Boiling point: unknown
Bohrium ![]()
Melting point: unknown
Boiling point: unknown
Hassium ![]()
Melting point: unknown
Boiling point: unknown
Meitnerium ![]()
Melting point: unknown
Boiling point: unknown
Darmstadtium ![]()
Melting point: unknown
Boiling point: unknown
Roentgenium ![]()
Melting point: unknown
Boiling point: unknown
Copernicium ![]()
Melting point: unknown
Boiling point: unknown
Nihonium ![]()
Melting point: unknown
Boiling point: unknown
Flerovium ![]()
Melting point: unknown
Boiling point: unknown
Moscovium ![]()
Melting point: unknown
Boiling point: unknown
Livermorium ![]()
Melting point: unknown
Boiling point: unknown
Tennessine ![]()
Melting point: unknown
Boiling point: unknown
Oganesson ![]()
Melting point: unknown
Boiling point: unknown
Alkali metals

Alkaline earth metals

Nitrogen group
(Pnictogens)

Oxygen group
(Chalcogens)

Halogens

Noble gases

Typical properties:
- high melting points
- high boiling points
- good electrical conductors
- good thermal conductors
- shiny when cut
- strong and malleable
Elements with properties in between
Typical properties:
- low melting points
- low boiling points
- poor electrical conductors
- poor thermal conductors
- dull
- brittle when solid
Physical state at room temperature: solid (s), liquid (l) or gas (g).
The weighted mean mass of the atoms
The number of protons in an atomic nucleus.
Also called the proton number.
Main differences between exam boards
| AQA | Edexcel | OCR | |
|---|---|---|---|
| Hydrogen, H | Immediately above Li | ||
| Atomic number | Above chemical symbol | ||
| Lanthanoids and actinoids | La and Ac are in the two white boxes | La and Ac are in the two white boxes | |
| Elements 112 – 118 | None shown | Cn, Fl, Lv only |