States of matter

Aims of this section
After studying this page, you should be able to:
- recall the names of the three states of matter and their interconversions
- describe the arrangement, movement and the relative energy of particles in the three states of matter
- explain what happens to particles during state changes
- predict the physical state of a substance under given conditions.
The particle model
The three common states of matter are solid, liquid and gas. The particle model describes how the particles of a substance are arranged and move in each state.
| Solid | Liquid | Gas | |
|---|---|---|---|
| Relative distance apart | Very close | Close | Far apart |
| Arrangement | Regular | Random | Random |
| Movement | Vibrate about fixed positions | Move around each other | Fast in all directions |
| 2D diagram |
The word ‘matter’ describes all the ‘stuff’ that is made of particles and which has mass. This includes you and all the substances and objects around you.
The diagrams do not show the movement of the particles, the forces between them or their stored energy. Remember that there is empty space between particles, and that these are just 2D diagrams of 3D situations.
Changes of state
The diagram summarises the four main changes of state.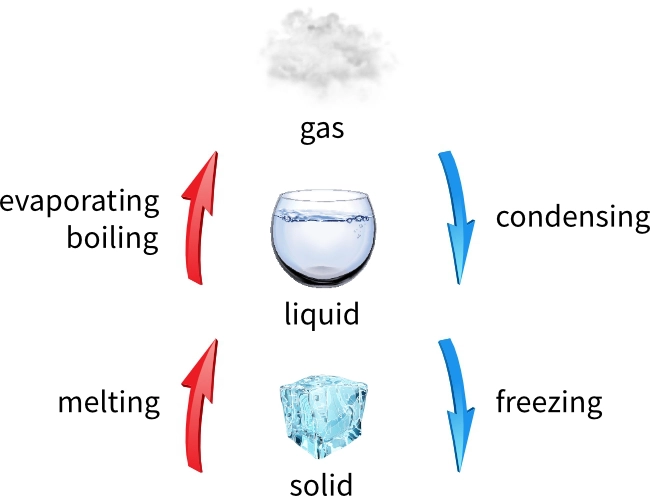
Some particles may have enough energy to escape from a substance in its liquid state. Evaporating only happens at the surface of the substance, but boiling happens all through the substance. Boiling occurs at or above the boiling point of the substance.
Two changes of state (not shown in the diagram) do not involve the liquid state:
- sublimation: solid → gas
- deposition: gas → solid
The video shows ‘dry ice’ (solid carbon dioxide) subliming at room temperature. It plays at 10× normal speed so you can see more clearly how the solid disappears without becoming a liquid.
Attractive forces can exist between particles. These can be overcome or broken by heating. When energy is transferred from the surroundings to the substance:
- some of the forces are overcome during melting
- all the remaining forces are overcome during evaporation or boiling.
When energy is transferred from the substance to the surroundings:
- some forces form during condensing
- many forces form during freezing.
The temperature of the substance stays the same during a state change.
Different attractive forces can exist between different types of particle. These include:
- covalent bonds between atoms
- intermolecular forces between simple molecules
- ionic bonds between oppositely charged ions.
Predicting physical states at different temperatures
The melting point of a substance is the temperature at which it melts or freezes. The boiling point of a substance is the temperature at which it boils or condenses. You can predict the state of a substance at a given temperature if you know its melting point and boiling point. The diagram shows how this works.
Fruit frozen in liquid nitrogen
Worked examples
The melting point of substance X is –7.2 °C and its boiling point is 58.8 °C.
Predict its state at:
(a) 25 °C
(b) –25 °C
(c) 75 °C
(a) Liquid (25 °C is between the melting point and boiling point)
(b) Solid (–25 °C is less than the melting point)
(c) Gas (75 °C is greater than the boiling point)
Steel in its liquid state
