Development of the periodic table

Aims of this page
After studying this page, you should be able to:
- describe the limitations of early periodic tables
- explain how Mendeleev overcame some of these limitations
- explain the importance of using the periodic table to make predictions
- explain why the existence of isotopes affected the order of some elements in periodic table.
Overview
Some key discoveries helped in the development of the periodic table, including:
- recognising that certain elements have some chemical properties in common
- determining accurate atomic weights
- realising that there were elements still to be discovered
- discovering the significance of proton number and the existence of isotopes.
Periodic table development – later work
The atoms of each element have different numbers of protons. The modern periodic table places elements in order of increasing atomic number (proton number).
Older periodic tables placed the elements in order of increasing atomic weight. The existence of isotopes made it possible to explain why it was not always correct to do this.
Döbereiner's triads
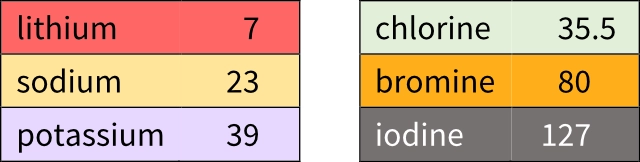
Around two hundred years ago, a German chemist called Johann Döbereiner discovered patterns in the properties of some elements. He placed sets of three elements with similar chemical properties in order of increasing atomic weight. The image shows two of these ‘triads’.
Pros ✓
Döbereiner noticed that the atomic weight of the middle element in each triad was equal to the mean of the other two elements.
For example, looking at the left-hand triad:
\(\ce\displaystyle\textsf{mean atomic weight} = \frac{\large\textsf{7 + 39}}{\large\textsf{2}} = \frac{\large\textsf{46}}{\large\textsf{2}} = \textsf{23}\)
You should see that this is the same as the atomic weight of sodium.
Döbereiner found a link between a physical property (atomic weight) and chemical properties (how the elements react with other elements).
This method was not so accurate for other triads. Check for yourself that the mean atomic weight of chlorine and iodine is not the same as the atomic weight of bromine.
Cons ✗
Döbereiner could not explain why elements should form triads, or the links between atomic weights in each triad. As time went by, more elements were discovered and this increased the numbers of elements in each triad to four or more.
The two triads shown consist of elements from modern groups 1 and 7. These groups contain more than three elements.
Newlands' octaves
An English chemist called John Newlands developed a table during the 1860s. Like Döbereiner, he placed the elements in order of increasing atomic weight. By 1865 his table showed the known elements arranged into eight columns, each containing seven elements. The numbers in his table refer to the positions of the elements, and are not their atomic weights.
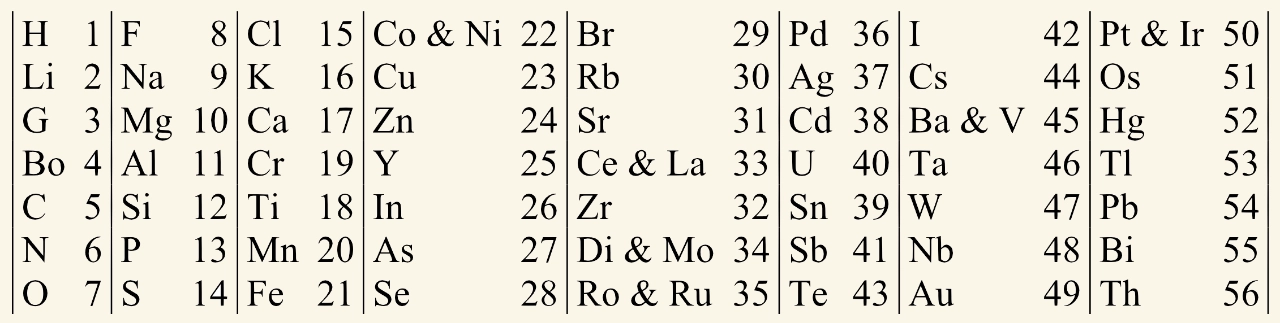
Only 62 elements were known when Newlands devised his table (118 are known today). Some of the element symbols he used have changed since then:
- G is Be, beryllium
- Bo is B, boron
- Ro is Rh, rhodium
Newlands noticed that every eighth element was similar in its chemical and physical properties, just like notes on a musical scale. For example, looking at the first row, you can see these elements:
fluorine, chlorine, bromine, iodine
They are found in that order in group 7 of the modern periodic table. You can see a similar situation in other rows. Row 2 contains elements found in group 1:
lithium, sodium, potassium, rubidium, caesium
Newlands included the first element in his counting. For example, starting with F and working downwards:
- F was counted as element 1
- Na was counted as element 2
- going down to S as element 7
- Cl at the top of the next column was element 8.
Pros ✓
Newlands discovered repeating or ‘periodic’ properties in the known elements.
Cons ✗
The patterns in Newlands’ table did not work well past calcium, Ca. For example:
- copper (a relatively unreactive metal) is in the same row as potassium and rubidium (very reactive metals)
- iron (a metal) is in the same row as oxygen and sulfur (non-metals).
Newlands’ work was heavily criticised by other chemists at the time. One even suggested that it might be better to arrange the elements alphabetically. His table was only published several months after he announced it because the work was highly theoretical.
Mendeleev's tables
A Russian chemist called Dmitri Mendeleev developed increasingly detailed tables, starting in 1869. Like Döbereiner and Newlands, he placed the elements in order of increasing atomic weight. However, Mendeleev:
- used the most accurate atomic weights available at the time
- left gaps where he thought there should be an undiscovered element.
Mendeleev also changed the places of some elements if doing so matched their properties better. Newlands did this too. If you look back at Newlands’ 1865 table, you should see that:
- Te is element 43 (when it should be numbered 42)
- I is element 42 (when it should be numbered 43).
Mendeleev was writing Russian chemistry books. He realised that he needed a good way to organise them, and his table was the key to doing this. Mendeleev devised his first table in just one day, but he had been working on the problem on and off for years.
1869 table
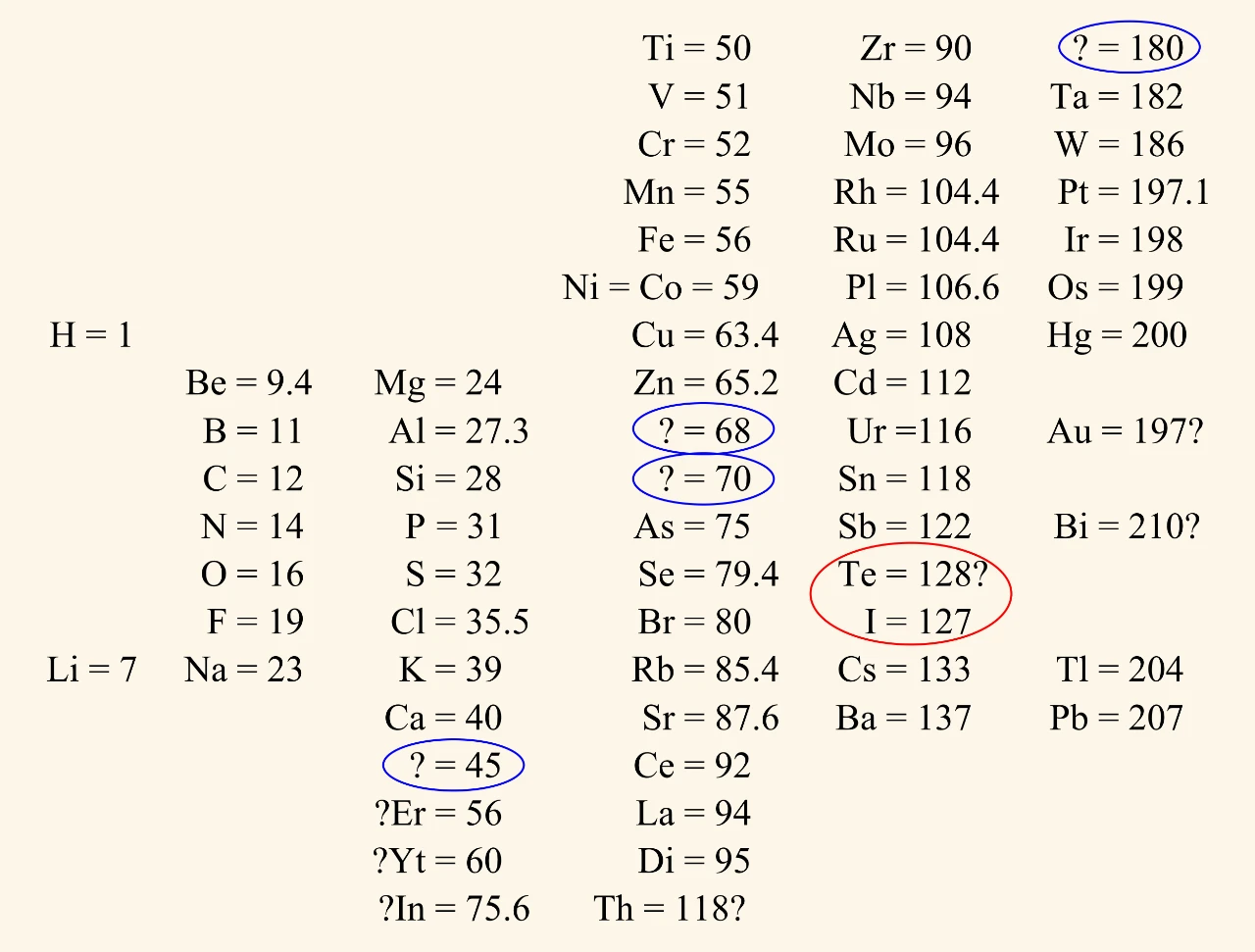
The gaps for undiscovered elements are circled in blue in this diagram.
Tellurium and iodine are examples of where Mendeleev swapped the positions of elements, like Newlands did. They are circled in red in this diagram.
The substance near the bottom with the symbol Di was called ‘didymium’. It was discovered in 1841 but by 1864 chemists thought it was actually a mixture. ‘Didymium’ was separated into two elements (praseodymium and neodymium) in 1885. Newlands and Mendeleev could not have known this when they devised their tables.
Mendeleev’s 1869 table differed from Newlands’ table:
- each column contained a different number of elements
- the numbers shown were atomic weights
- gaps existed where Mendeleev thought undiscovered elements should be.
1871 table
Mendeleev continued to work on his table. By 1871, his table:
- was rotated through 90°
- included general formulae for the oxides and hydrides formed by elements.
His 1871 table also resembled the modern periodic table:
- eight vertical groups containing elements with similar chemical properties, such as the formula of their common compounds with oxygen and hydrogen
- horizontal ‘series’ (called periods in the modern table).
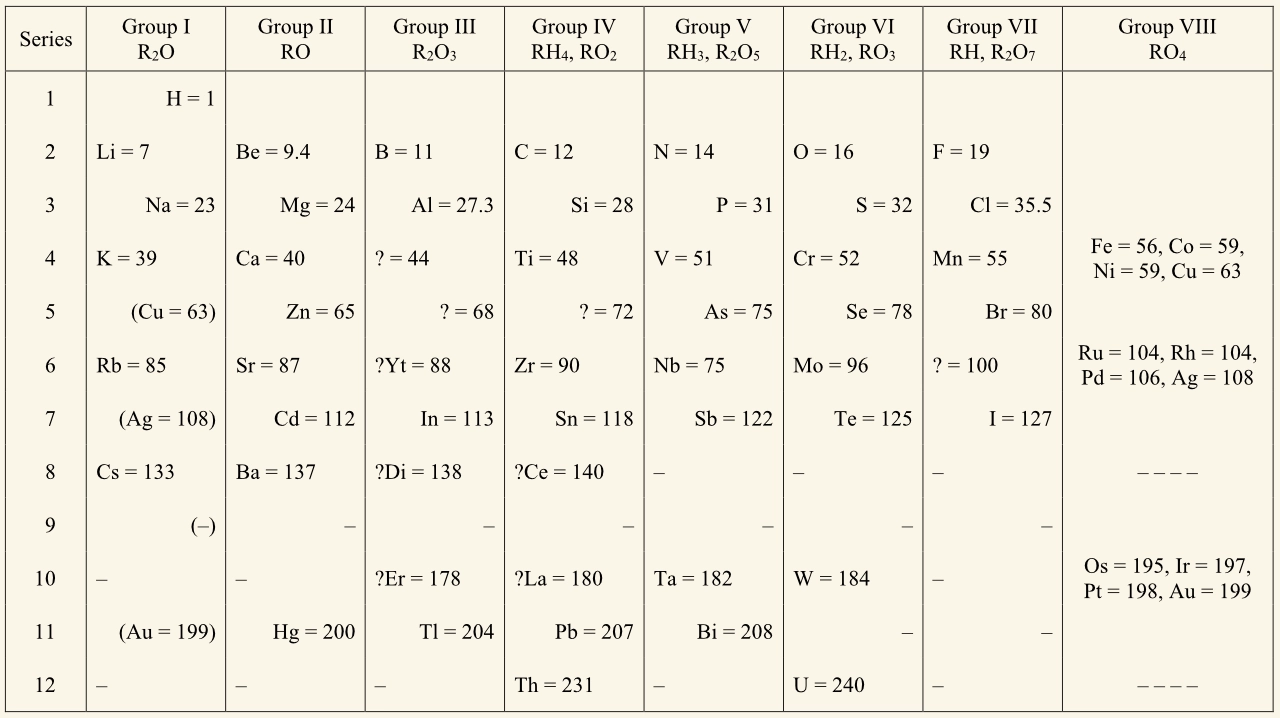
Mendeleev’s 1871 table looked similar to the modern periodic table, but had differences such as:
- the group on the far right held iron and other transition metals, rather than the noble gases
- the transition metals were not placed in a block of their own
- there were no atomic numbers.
Pros ✓
Mendeleev’s groups contained elements with similar chemical properties.
His gaps did two things:
- they solved Newlands’ problem where elements with different properties were in the same ‘octave’
- they allowed Mendeleev to make predictions about undiscovered elements that might fit in those gaps.
The noble gases were discovered between 1895 and 1900. They were place on the right-hand side of the periodic table, showing that the table was adaptable enough to fit in a whole new group.
Cons ✗
Mendeleev could not explain why he needed to swap the positions of tellurium and iodine, other than this made his table work properly. He was so convinced it was the right thing to do, he thought that the atomic weight of tellurium must be wrong. He altered its value to keep the order of increasing weights intact.
Mendeleev actually showed the correct atomic weights for these two elements in his 1869 table. He had no experimental evidence for changing the atomic weight of tellurium from 128 to 125.
Mendeleev's predictions
The prefix eka- comes from the Sanskrit word for 1. Mendeleev meant it to show that eka-aluminium was one place down from aluminium in the same column.
| Property | Eka-aluminium, Ea (predicted) | Gallium, Ga (actual) |
|---|---|---|
| Atomic weight | 68 | 69.7 |
| Density of element /g cm–3 | 6.0 | 5.9 |
| Formula of oxide | Ea2O3 | Ga2O3 |
| Density of oxide /g cm–3 | 5.5 | 5.9 |
| Soluble in acids? | ✓ | ✓ |
| Soluble in alkalis? | ✓ | ✓ |
Mendeleev made predictions about 16 undiscovered elements. Half of these elements were found later and half were never found. We now know that two of them could not exist because their predicted atomic weights were less than 1 (the atomic weight of hydrogen).
Mendeleev’s table allowed fairly accurate predictions about elements that were discovered later. This was powerful evidence in favour of it.
Future discoveries about atomic structure were needed to complete the table.
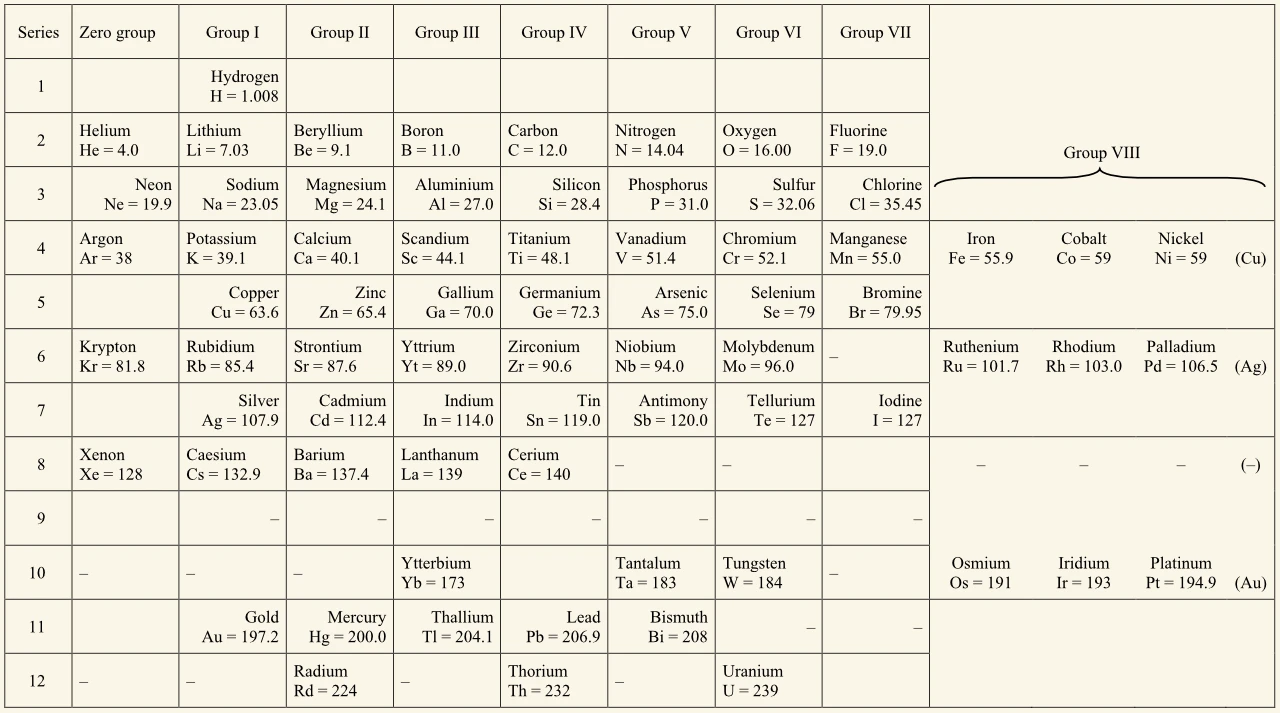
Mendeleev's 1904 periodic table
The noble gases were discovered between 1895 and 1900. Compared to his earlier tables, Mendeleev’s 1904 table:
- showed noble gases on the left-hand side in a ‘zero group’
- more accurate and precise atomic weights.
There were similarities, including:
- transition elements were in a ‘group VIII’ on the right-hand side
- elements were arranged in order of increasing atomic weight
- the positions of some elements, such as iodine and tellurium, were changed to suit their properties better.
Hydrogen was the standard element in the 19th century with an atomic weight of 1, but in the first half of the 20th century the standard element was oxygen with an atomic weight of 16.
Isotopes
Atoms of an element that have different numbers of neutrons are called isotopes. This means that isotopes of an element have the same atomic number but different mass numbers (total numbers of protons and neutrons). The existence of isotopes is why Mendeleev could not explain why he changed the positions of tellurium and iodine.

Naturally occurring iodine has just one isotope, 127I. However, naturally occurring tellurium has eight isotopes. Of these, about 32% is 128Te and 34% is 130Te. The masses of these isotopes are enough to make the Ar of tellurium greater than the Ar of iodine, even though its atomic number of tellurium is less than that of iodine.
The existence of isotopes was first suggested by Frederick Soddy in 1913. Mendeleev had died six years earlier.
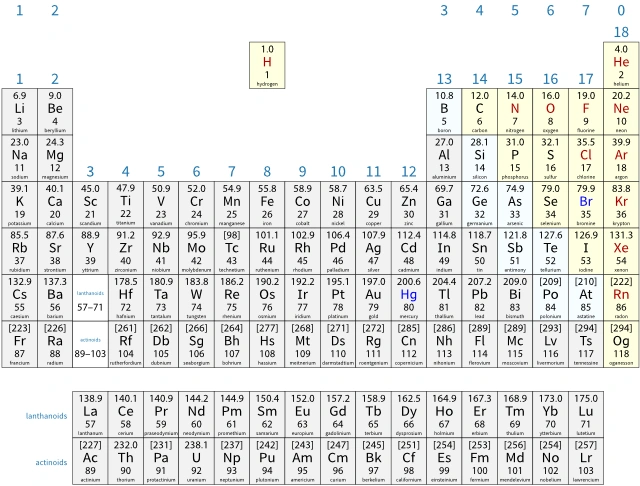
The modern periodic table
In the modern periodic table:
- an atomic number is the number of protons in the nucleus of an atom
- elements are arranged in order of increasing atomic number
- noble gases are placed on the right-hand side
- transition metals are placed in the middle.
Periodic tables show relative atomic masses, Ar, rather than atomic masses. These are the weighted mean mass of the atoms of each element, compared to one-twelfth the mass of a carbon-12 atom. The 12C atom is the standard atom today. Its Ar is defined as 12 exactly.
Periodic table development
The atoms of each element have different numbers of protons. The modern periodic table places elements in order of increasing atomic number (proton number).
Older periodic tables placed the elements in order of increasing atomic weight. The existence of isotopes made it possible to explain why it was not always correct to do this.
creative-chemistry.org.uk
