The periodic table
Aims of this section
After studying this section, you should be able to:
- describe how the periodic table developed from early ideas
- explain how the elements are organised in the modern periodic table
- predict the electronic configurations of the first twenty elements
- identify the positions of metals and non-metals in the table
- describe typical properties of metal and non-metal elements.
Summaries
Development of the periodic table
Döbereiner's triads
Johann Döbereiner discovered patterns in the properties of some elements. He placed sets of three elements with similar chemical properties in order of increasing atomic weight.
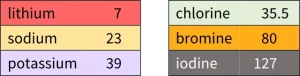
Döbereiner discovered that the atomic weight of the middle element in a triad was equal to the average atomic weight of the other two elements.
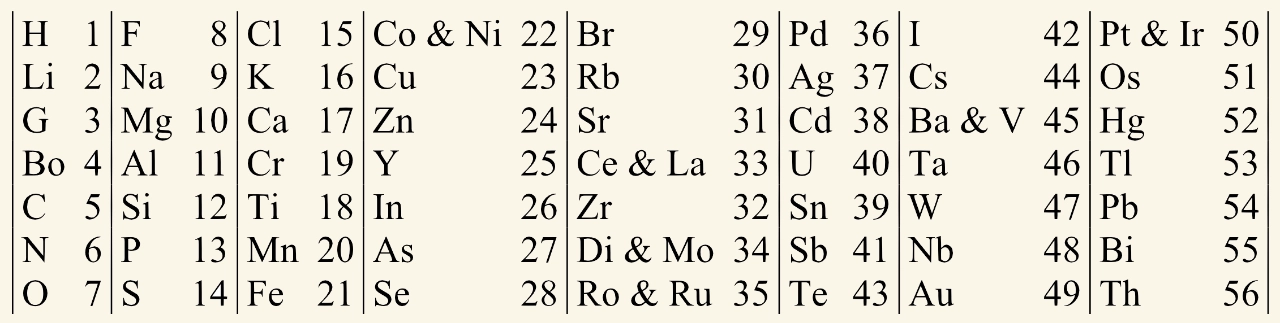
Newlands' octaves
John Newlands placed the known elements into eight columns, each containing seven elements. He noticed that every eighth element was similar in its chemical and physical properties. However, this pattern did not work past calcium.
Mendeleev's tables
Dmitri Mendeleev developed increasingly detailed tables, starting in 1869. Like Döbereiner and Newlands, he placed the elements in order of increasing atomic weight. However, Mendeleev:
- used the most accurate atomic weights available at the time
- left gaps where he thought there should be an undiscovered element.
Mendeleev also changed the places of some elements if doing so matched their properties better. He did this with tellurium, Te, and iodine, I.
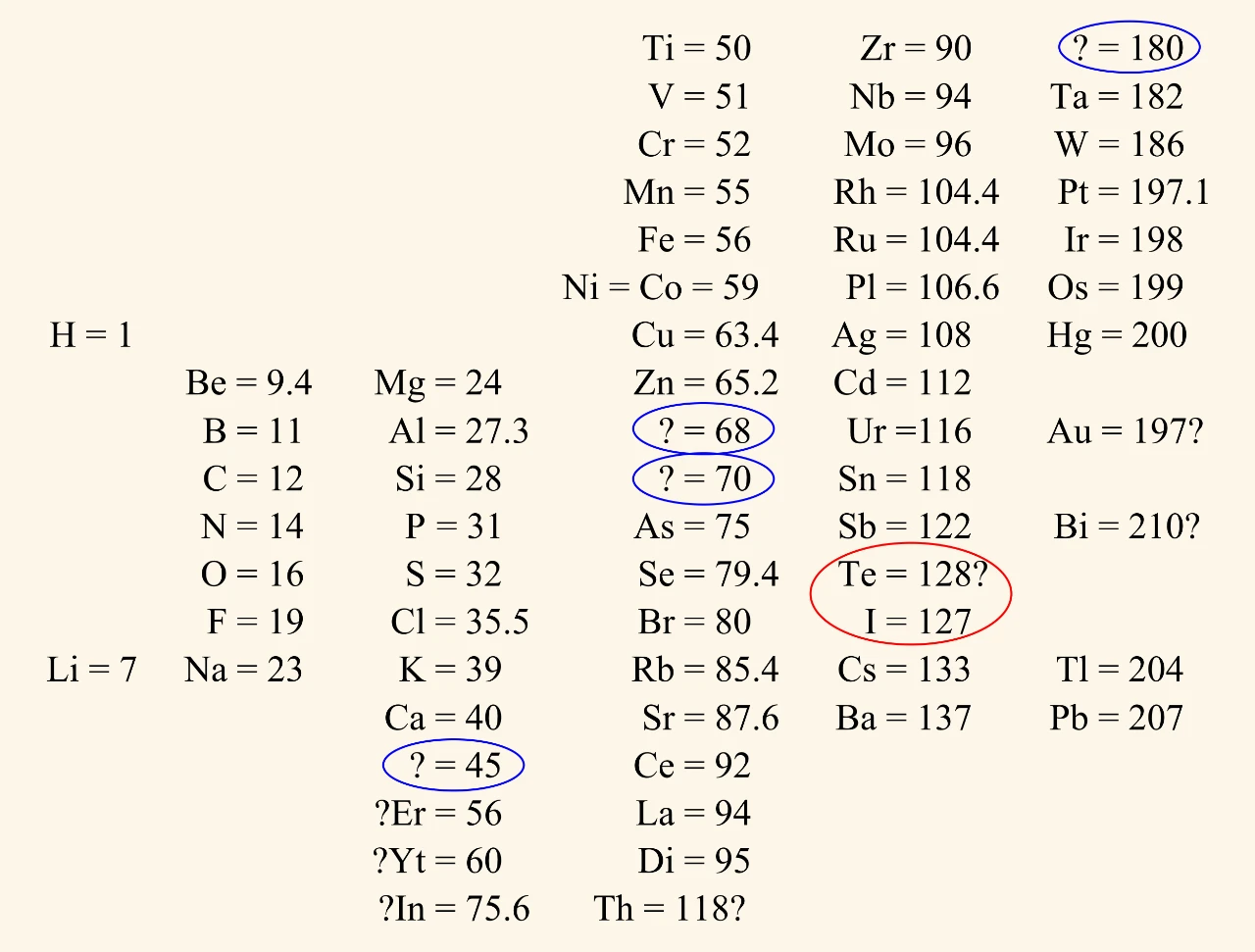
Mendeleev could not explain why a strict order of atomic weight should not always work. The discovery of isotopes in the 20th century solved this problem.
Naturally occurring iodine has just one isotope, 127I. However, naturally occurring tellurium has eight isotopes, including 128Te and 130Te. The masses of these isotopes are enough to make the atomic weight of tellurium greater than that of iodine.
Mendeleev's predictions
Mendeleev used his table to predict the properties of elements that were unknown at the time. When these elements were discovered later, their properties were similar to their predicted properties. Mendeleev’s table allowed fairly accurate predictions about elements that were discovered later. This was powerful evidence in favour of it.
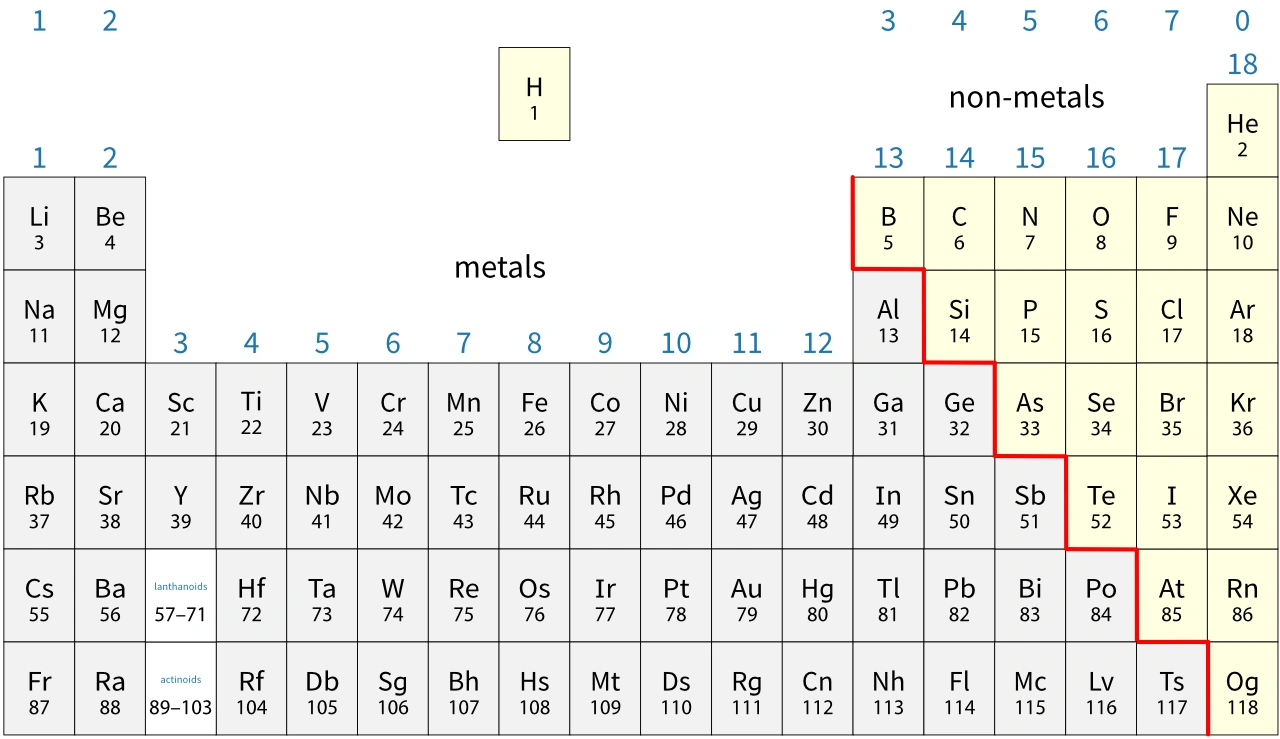
The modern periodic table
In the modern periodic table:
- an atomic number is the number of protons in the nucleus of an atom
- elements are arranged in order of increasing atomic number.
Periodic tables show relative atomic masses, Ar, rather than atomic masses. These are the weighted mean mass of the atoms of each element, compared to one-twelfth the mass of a carbon-12 atom. The 12C atom is the standard atom today. Its Ar is defined as 12 exactly.
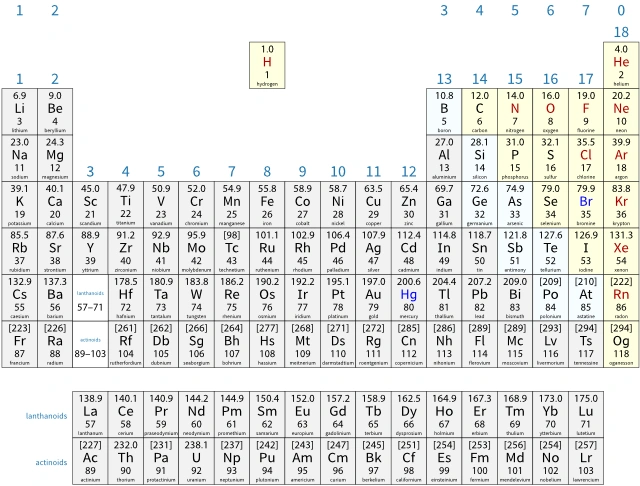
Electronic configurations
Electrons are arranged in shells around the nucleus. Different shells can contain different numbers of electrons. An electronic configuration describes the way in which electrons in an atom are arranged in these shells. For example, the electronic configuration for sodium (atomic number 11) is:
2.8.1
This shows that 2 electrons are in the first shell, 8 in the second shell, and 1 electron in the outer shell. You can also tell from this electronic configuration that sodium:
- is placed in group 1, period 3
- has (2 + 8 + 1) = 11 electrons in its atoms.
You may see electronic configurations described as electron arrangements, or written with commas rather than full stops. You may use the term ‘energy level’ instead of ‘shell’.
Metals and non-metals
The general properties of metals and non-metals are different. In a rough sense they are opposites of each other. Metals are found to the left of the periodic table and non-metals to the right. This means that the properties of elements are related to their electronic configurations.