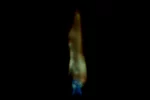
Flame tests

Learning outcomes
After studying this page, you should be able to:
- describe how to do flame tests
- recall the flame colours for these ions:
- lithium, Li+
- sodium, Na+
- potassium, K+
- copper(II), Cu2+
- calcium, Ca2+
- barium, Ba2+
Background information
Metal atoms and their ions give off light when they are heated in a hot flame. Some of this light is in the visible spectrum, and it changes the colour of the flame.
The colour you see depends upon the metal and the temperature of the flame. You can work out which metal ions are in a sample if you know the characteristic colour they produce.
Flame tests are easy to do but you do need to work carefully as usual. They work with samples in solution and with samples in the solid state.
The nucleus in an atom is surrounded by electrons in energy levels. Electrons can move to higher energy levels when they absorb energy, and they emit energy when they move to lower energy levels. The difference in energy determines the wavelength of light absorbed or emitted.
Method
You need to use a hot (blue) Bunsen burner flame (not the luminous orange safety flame). A common method uses a nichrome wire loop attached to a handle:
- Clean the loop by dipping it into hydrochloric acid, then holding it at the edge of the flame. You may need to do this more than once.
- Dip the cleaned loop in the sample.
- Hold the loop at the edge of the flame.
- Observe and record the flame colour you see.
You can also use a wooden splint soaked in a sample solution, or a damp wooden splint to pick up a solid sample. The videos below show this being done.

Results
| Metal ion | Flame test colour |
|---|---|
| lithium, Li+ | red |
| sodium, Na+ | yellow |
| potassium, K+ | lilac |
| calcium, Ca2+ | orange-red |
| copper(II), Cu2+ | blue-green |
| barium, Ba2+ | light green |
Flame tests do not work well with mixtures. The different colours just merge together, so you cannot easily work out what is in the sample.
The orange flame colour of sodium is very intense. It often masks the colours from other metals, so you must make sure you clean the loop properly.
Videos of flame tests
- Flame testing salts of lithium, sodium, potassium, calcium, copper(II) and strontium.
- Flame testing lithium chloride
- Flame testing sodium chloride
- Flame testing potassium chloride
- Flame testing calcium chloride
- Flame testing copper chloride
- Flame testing strontium chloride
- Reaction of calcium carbonate with acid, testing for sulfate ions, and testing for iodide ions.