Electronic configurations
Aims of this page
After studying this page, you should be able to:
- predict the electronic configurations of the first 20 elements
- explain how an element’s electronic configuration is linked to its position on the periodic table.
What are electronic configurations?
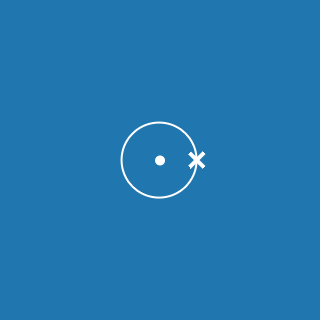
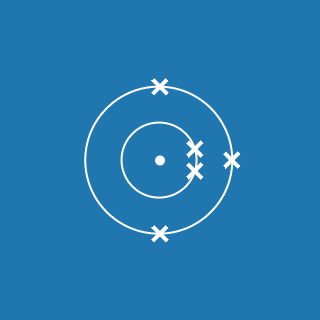
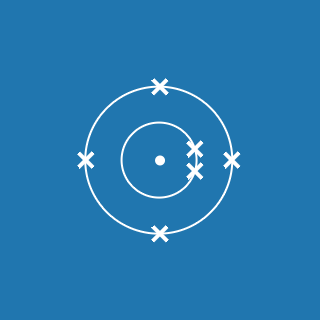
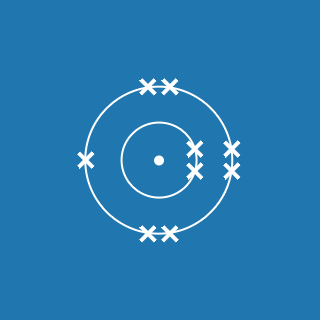
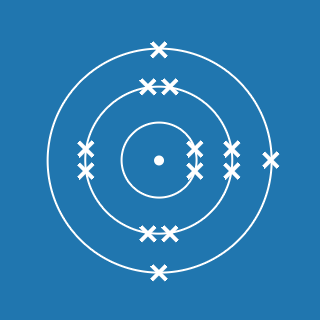
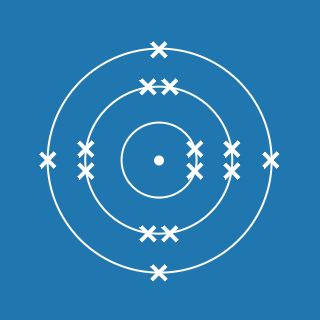
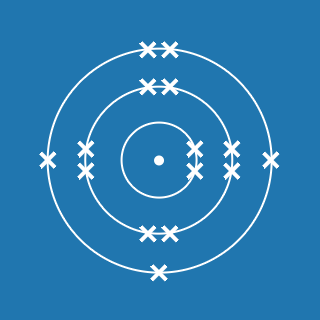
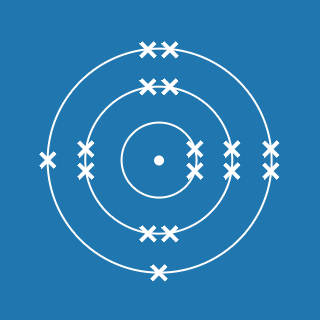
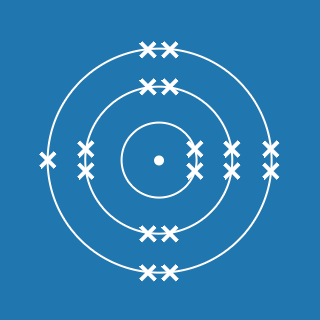
2.8.1
You may see electronic configurations written with commas instead of full stops.
This electronic configuration tells you that:
- the first shell contains 2 electrons
- the second shell contains 8 electrons
- the outer shell contains 1 electron
- there are three occupied shells
- the total number of electrons is (2 + 8 + 1) = 11.
Remember that atoms contain equal numbers of protons and electrons. A sodium atom contains 11 electrons and 11 protons (and its atomic number will be 11).
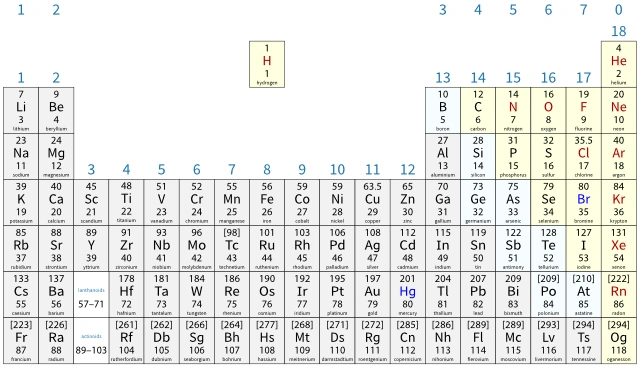
The section of the periodic table here shows the first 20 elements. The gap between groups 2 and 3 (IUPAC groups 2 and 13) has been removed.
You should see that:
- the number of occupied shells is equal to the period number
- the number of electrons in the outer shell is equal to the group number (except for group 0, where the outer shell is full).
1
2
2.1
2.2
2.3
2.4
2.5
2.6
2.7
2.8
2.8.1
2.8.2
2.8.3
2.8.4
2.8.5
2.8.6
2.8.7
2.8.8
2.8.8.1
2.8.8.2
Predicting electronic configurations
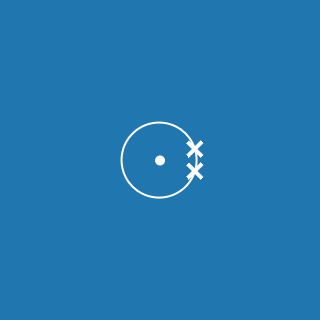
Different shells can hold different numbers of electrons.
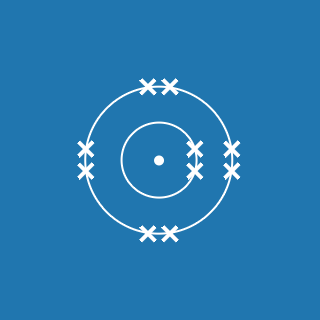
The table shows the maximum number of electrons the first three shells can hold. This takes you as far as argon (atomic number 18). Potassium and calcium atoms contain electrons in the fourth shell:
- K 2.8.8.1
- Ca 2.8.8.2
You need to be able to predict the electronic configurations of the first 20 elements (hydrogen to calcium). You can do this from the atomic number of the element or by using the periodic table.
| Shell | Maximum number of electrons |
|---|---|
| 1 | 2 |
| 2 | 8 |
| 3 | 8 |
From an atomic number
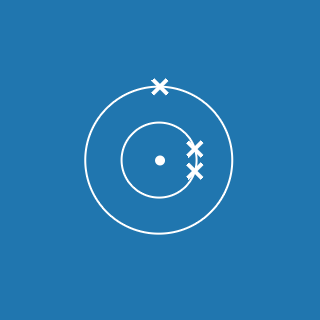
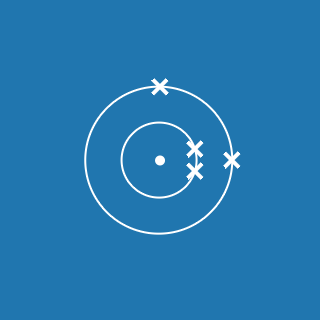
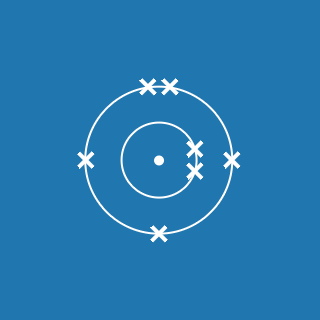
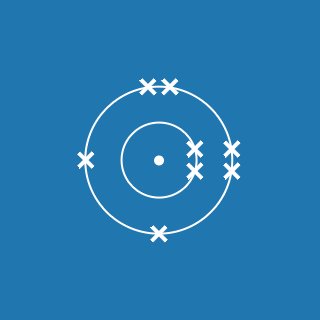
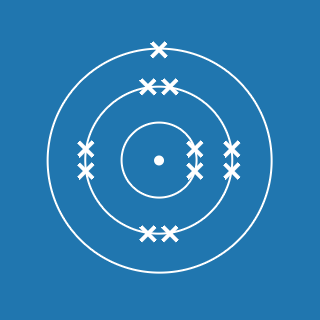
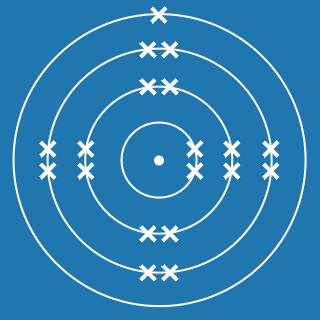
The atomic number of an element gives you the number of electrons its atoms contain. The atomic number of phosphorus is 15, so there are 15 electrons arranged around the nucleus:
- 2 will occupy shell 1, with 13 electrons left over
- 8 of the remaining electrons will occupy shell 2, with 5 electrons left over
- 5 electrons will occupy shell 3.
This means that the electronic configuration of phosphorus is 2.8.5 (the diagram is also shown here).
Quick checks
You will be given a copy of the periodic table to use in your exams. This means you can check your answer easily:
- phosphorus is in period 3, so it will have 3 occupied shells (three numbers or three circles in its predicted electronic configuration)
- phosphorus is in group 5, so it will have 5 electrons in its outer shell (the last number in its electronic configuration is 5)
- the total number of electrons is (2 + 8 + 5) = 15 (the atomic number of phosphorus).
Just make sure that no number is greater than 8, and that the first number is 2 (unless it is hydrogen).
Worked example 1
The electronic configuration of neon is 2.8. State and explain the group and period where neon is found.
Neon belongs to group 0. This is because its atoms have a full outer shell. Neon is found in period 2 because its atoms have 2 occupied shells of electrons.
Worked example 2
The electronic configuration of silicon is 2.8.4. State and explain the atomic number of silicon.
The atomic number of silicon is 14. This is because its atoms contain (2 + 8 + 4) = 14 electrons, so they also contain 14 protons. The atomic number of an element is equal to the number of protons its atoms contain.
If your GCSE specification uses the IUPAC group numbers 1 to 18, you will need to make adjustments. For group numbers 13 to 18:
- subtract 10 to go from group number to number of outer electrons
- add 10 to go from number of outer electrons to group number.
Using the periodic table
The electronic configuration of an element and its position in the periodic table are related. This gives you another way to predict electronic configurations.
Worked example 3
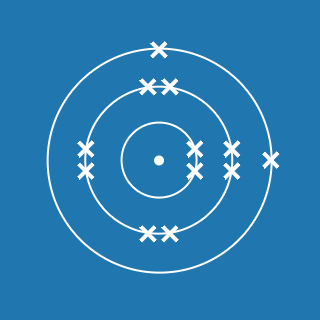
Chlorine is placed in period 3 and group 7 in the periodic table. Predict its electronic configuration. Explain your answer.
2.8.7
This is because:
- it will have 3 occupied shells (the number of numbers)
- the outer shell will contain 7 electrons (the group number)
- shell 1 can contain 2 electrons
- shell 2 can contain 8 electrons.
You can predict an electronic configuration by counting across the periodic table:
- find the target element
- count from left to right across each period in turn, starting with hydrogen, until you reach your target element.
For chlorine:
- 2 elements H to He
- 8 elements Li to Ne
- 7 elements Na to Cl.