Molecular models
What is here?
To help you understand organic chemistry better, I have put together some molecular models for you to play with. You can move, rotate and resize them, and change the type of model shown.
If you can see the rotating model of a section of graphyne-1, you’re in business!
-
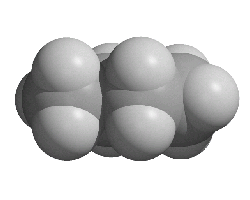 Alkanes
Alkanes
-
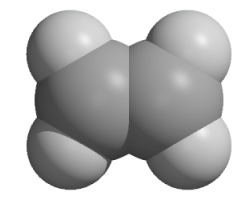 Alkenes
Alkenes
-
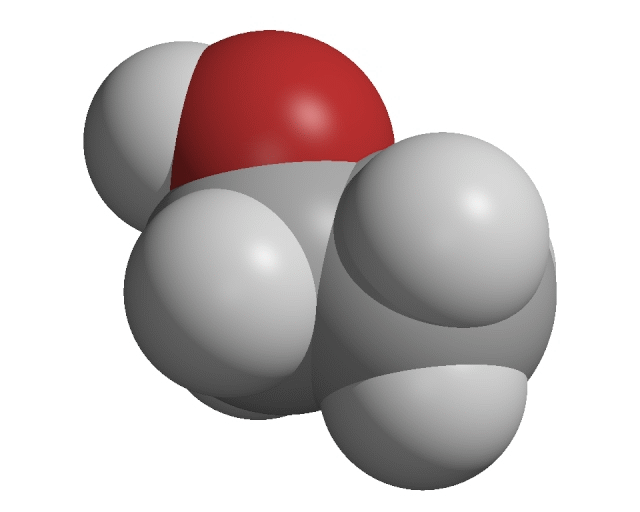 Alcohols
Alcohols
-
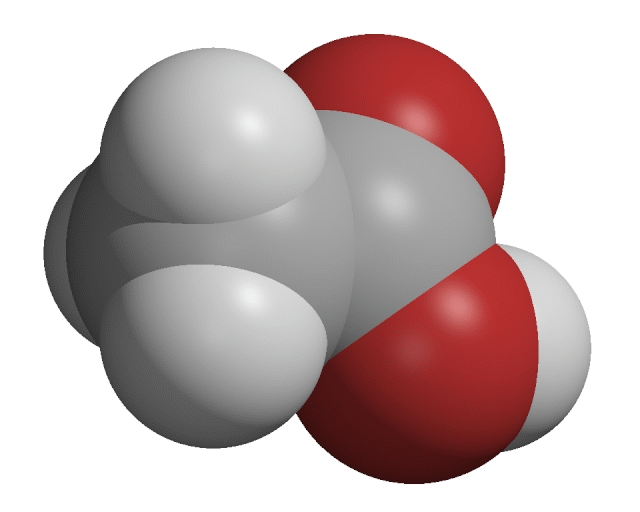 Carboxylic acids
Carboxylic acids
-
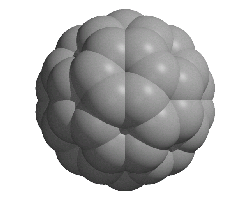 Carbon allotropes
Carbon allotropes
-
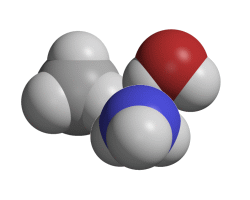 Tetrahedral molecules
Tetrahedral molecules
-
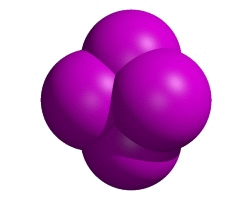 Trigonal bipyramidal
Trigonal bipyramidal
-
 Octahedral molecules
Octahedral molecules
-
 Isomerism
Isomerism
The alkanes are a homologous series of hydrocarbons. Carbon atoms in alkane molecules are joined by single covalent bonds.
Creative Chemistry has molecular models of the first four unbranched alkanes.
The alkenes are a homologous series of hydrocarbons. Alkene molecules contain a carbon-carbon double bond, C=C.
Creative Chemistry has molecular models of unbranched alkenes with 2, 3 or 4 carbon atoms.
The alcohols are a homologous series of organic compounds. Alcohol molecules contain a hydroxyl group, −OH.
Creative Chemistry has molecular models of simple alcohols with 1 to 4 carbon atoms.
The carboxylic acids are a homologous series of organic compounds. Carboxylic acid molecules contain a carboxyl group, −COOH.
Creative Chemistry has molecular models of simple carboxylic acids with 1 to 4 carbon atoms.
Allotropes are different structural modifications of an element.
Creative Chemistry has molecular models of graphene, graphite, a carbon nanotube, a buckyball and diamond.
These molecules have four pairs of outer electrons around their central atom.
Creative Chemistry has molecular models of methane, ammonia and water.
These molecules have five pairs of outer electrons around their central atom.
Creative Chemistry has molecular models of phosphorus pentafluoride, sulfur tetrafluoride and chlorine trifluoride.
These molecules have six pairs of outer electrons around their central atom.
Creative Chemistry has molecular models of sulfur hexafluoride, iodine pentafluoride and xenon tetrafluoride.
Isomers are molecules with the same molecular formula but different arrangements of atoms.
Choose from these types of isomerism.
Chain isomers have the same molecular formula, but the way their carbon atoms are joined together differs from isomer to isomer. Position isomerism occurs when a functional group can occupy different positions on the same carbon chain. Functional group isomerism occurs when substances have the same molecular formula but different functional groups Stereoisomerism occurs when substances have the same molecular formula, but a different arrangement of their atoms in space. Cis-trans isomerism is one type of this isomerism. The E-Z naming system for geometrical isomers is more flexible than the cis-trans naming system. It is based on the positions of the groups attached to a C=C bond, with each group assigned a priority.
