Subatomic particles and isotopes

Aims of this page
After studying this page, you should be able to:
- explain what is meant by atomic number and mass number
- describe the use of isotope notation
- calculate the numbers of protons, neutrons and electrons in atoms and ions
- explain what isotopes are.
Atomic number and mass number
The diagram shows the standard nuclear notation for a sodium atom. You can see two numbers next to the chemical symbol:
- atomic number, the number of protons in the nucleus
- mass number, the total number of protons and neutrons in the nucleus.
It tells you that the nucleus contains:
- a total of 23 protons and neutrons
- 11 protons.
Subatomic particles in atoms
You can calculate the number of particles in an atom from these two numbers:
- number of protons = atomic number
- number of neutrons = (mass number) – (atomic number)
- number of electrons = number of protons
Worked example 1
The atomic number of aluminium is 13. Its mass number is 27. Calculate the numbers of protons, neutrons and electrons in an atom of aluminium.
Number of protons = 13
Number of neutrons = (27 – 13) = 14
Number of electrons = number of protons = 13
Subatomic particles in ions
Ions are charged particles formed when an atom (or group of atoms) gain or lose electrons:
- negative ions form when electrons are gained
- positive ions form when electrons are lost.
The nucleus does not change when an ion forms. The numbers of protons and neutrons stay the same. However, the number of electrons changes.
You can tell how many electrons are lost or gained from the number of charges.
Negative ions are called anions because they are attracted to the positively charged anode during electrolysis.
Positive ions are called cations (“cat-ions” not “cay-shuns”) because they are attracted to the negatively charged cathode during electrolysis.
Worked example 2
The atomic number of oxygen is 8. An oxygen atom gains 2 electrons to form an oxide ion, O2–. Calculate the number of electrons in the oxide ion.
The oxygen atom gains 2 electrons when it forms the oxide ion:
Number of electrons = atomic number + 2
= 8 + 2 = 10
Worked example 3
The atomic number of aluminium is 13. An aluminium atom loses 3 electrons to form an aluminium ion, Al3+. Calculate the number of electrons in the aluminium ion.
The aluminium atom loses 3 electrons when it forms the aluminium ion:
Number of electrons = atomic number – 3
= 13 – 3 = 10
Remember that electrons are negatively charged, so atoms that lose electrons become positively charged ions.
You may have noticed that the oxide ion and the aluminium ion both contain 10 electrons. Take care though:
- they have the same number of electrons as each other (and as a neon atom), but
- their nuclei are different, so they are not ions or atoms of the same element.
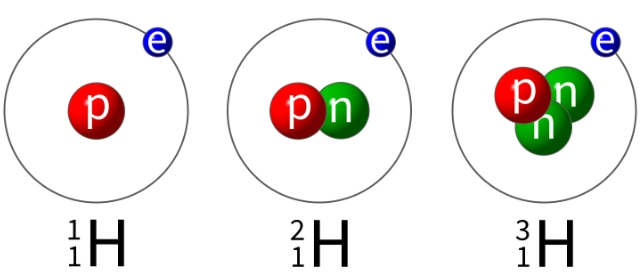
Isotopes
Isotopes of an element are different atoms with:
- the same number of protons but different numbers of neutrons in their nuclei
- the same atomic number but different mass numbers.
Isotopes of an element have identical chemical properties. This is because they have the same number of electrons (and so the same electronic configuration).
Worked example 4
The symbols for three different atoms are shown below. The letters in the symbols are not taken from the periodic table.
(a) \(\ce{^{13}_{7}X}\)
(b) \(\ce{^{13}_{6}Y}\)
(c) \(\ce{^{14}_{7}Z}\)
Identify two isotopes of the same element. Explain your answer.
Atom (a) and atom (c) are isotopes of the same element.
This is because they have the same atomic number (7), but different mass numbers (13 and 14). Both atoms have 7 protons, but atom (a) has 6 neutrons and atom (c) has 7 neutrons. Atom (b) only has 6 protons, so it belongs to a different element.
There are no “real”, “proper” or “actual” isotopes of an element. Some samples of an element may contain only one isotope, while others may contain two or more isotopes. Some of these isotopes may be found naturally and others may be artificial. Some isotopes are radioactive and others are not. Whatever they are like, all isotopes of an element react in the same way in chemical reactions.
