Reversible reactions

Aims of this page
After studying this page, you should be able to:
- give examples of reversible reactions
- describe and explain reversible reactions.
Forwards and backwards
This is a general equation for a reversible reaction:
A + B ⇌ C + D
The reaction between A and B is called the forward reaction. The reaction between C and D is called the reverse reaction (sometimes called the backward reaction).
Many reactions go to completion, which happens when one of the reactants is used up. You are likely to be familiar with these reactions. They include:
- fuels burning
- metals reacting with acids
- precipitation reactions.
Energy can be transferred into a reaction mixture by heating or by passing an electric current through it.
Examples
Ammonium chloride
Ammonium chloride is a white solid, and ammonia and hydrogen chloride colourless gases. They are involved in this reversible reaction:
\(\ce{NH4Cl(s)\ <=>[\ce{heating}][\ce{cooling}] {\ce{NH3(g) + HCl(g)}}}\)
The video shows ammonia and hydrogen chloride reacting together. The white fumes are clouds of ammonium chloride produced in the reaction.
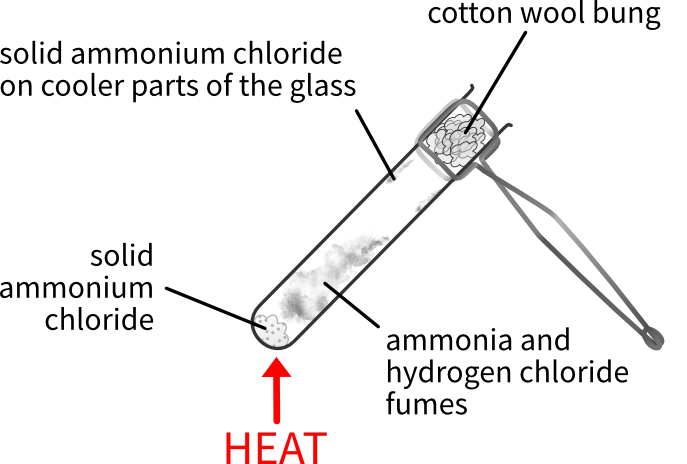
The reaction is easily investigated in the lab.
- Put some solid ammonium chloride into a test tube.
- Put a cotton wool bung into the mouth of the test tube (to stop alkaline and acidic fumes escaping into the lab).
- Heat gently to decompose the ammonium chloride.
You see solid ammonium chloride forming on the surface of the glass near to the cotton wool, where it is cool enough for the ammonia and hydrogen chloride to recombine.
Copper(II) sulfate
Hydrated copper(II) sulfate has a familiar deep blue colour.


Blue hydrated copper(II) sulfate becomes white anhydrous copper(II) sulfate when it is heated. This is because water is removed from the crystal lattice:
\(\ce{CuSO4\cdot5H2O <=>[\ce{endothermic (energy in)}][\ce{exothermic (energy out)}] {\ce{CuSO4 + 5H2O}}}\)
The reverse reaction releases a lot of energy. If you a few drops of water to anhydrous copper(II) sulfate, so much heating happens that the water boils.
Cobalt(IV) chloride
Anhydrous cobalt(IV) chloride is blue. It becomes pink hydrated cobalt(IV) chloride when water is added:
\(\ce{CoCl4 + 6H2O <=> {\ce{CoCl4\cdot6H2O}}}\)
The colour change from blue to pink is useful because you can test for the presence of water using cobalt chloride paper.
Cobalt chloride paper is white filter paper that has been soaked in cobalt chloride solution, then dried. The paper changes from blue to pink when it touches water vapour or a drop of water.


