Periodic table (GCSE)
What is here?
The periodic table below is based on the ones used by the different examination boards.
The group numbers 1 to 0 (the top ones) are used in most GCSE courses. The group numbers 1 to 18 were recommended by IUPAC in 1988. These are only used in OCR courses at the moment.
There is a summary at the bottom of the page. It shows the differences between this table and the ones used by three examination boards.
The lanthanoids and actinoids are not shown in GCSE periodic tables (only in A Level tables).
Select an element using your mouse (or finger on touch devices). You will see its melting and boiling points, electronic configuration (first 20 elements only), and if it is radioactive or toxic.
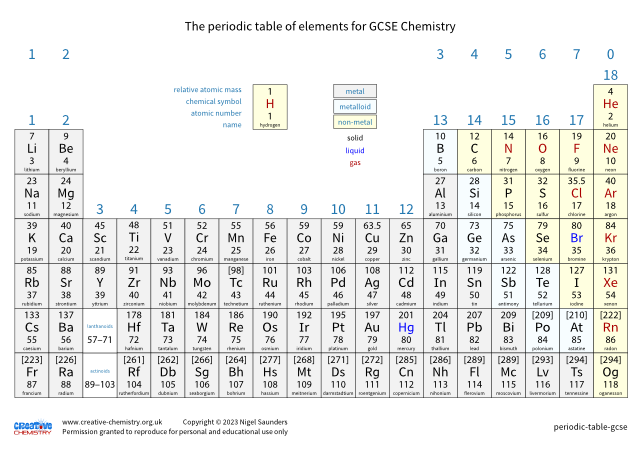
Hydrogen
Melting point: –259.2 °C
Boiling point: –252.9 °C
1
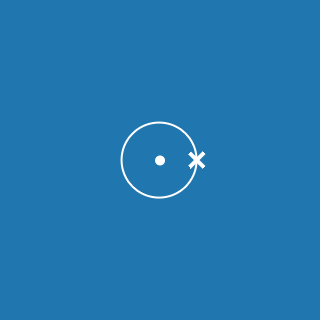
Helium
Melting point: –272.2 °C
Boiling point: –268.9 °C
2
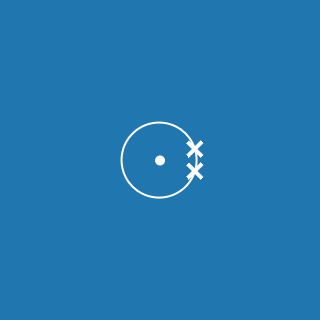
Lithium
Melting point: 180.5 °C
Boiling point: 1330 °C
2.1
Beryllium
Melting point: 1287 °C
Boiling point: 2469 °C
2.2
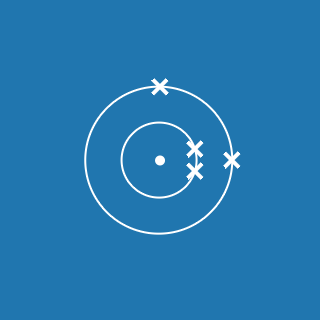
Boron ![]()
Melting point: 2076 °C
Boiling point: 3927 °C
2.3
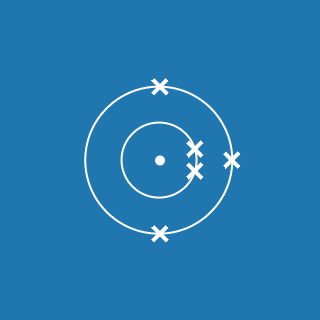
Carbon
Sublimes at 2642 °C
solid → gas
2.4
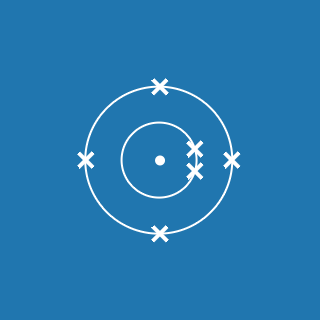
Nitrogen
Melting point: –210 °C
Boiling point: –195.8 °C
2.5
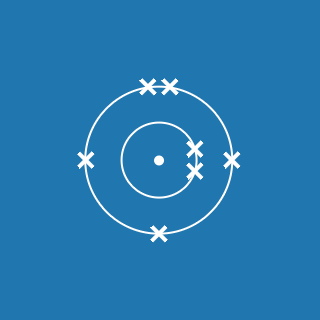
Oxygen
Melting point: –218.8 °C
Boiling point: –183 °C
2.6
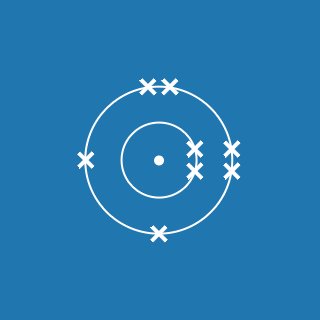
Fluorine ![]()
Melting point: –219.7 °C
Boiling point: –188.1 °C
2.7
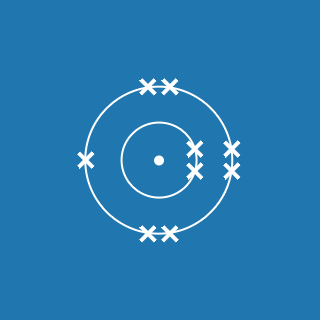
Neon
Melting point: –248.6 °C
Boiling point: –246.1 °C
2.8
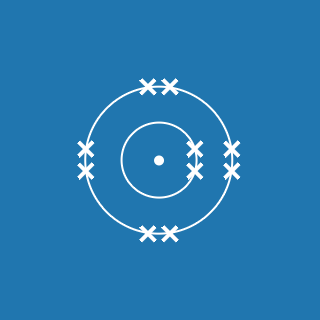
Sodium
Melting point: 97.8 °C
Boiling point: 882.9 °C
2.8.1
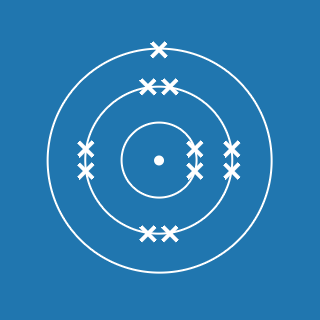
Magnesium
Melting point: 650 °C
Boiling point: 1091 °C
2.8.2
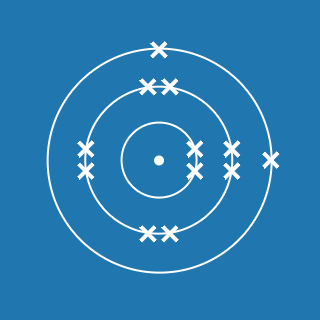
Aluminium
Melting point: 660.3 °C
Boiling point: 2470 °C
2.8.3
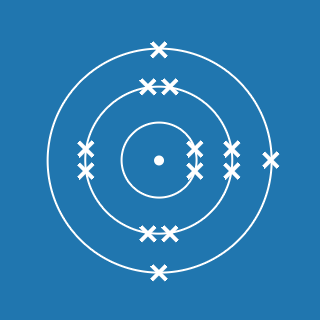
Silicon
Melting point: 1414 °C
Boiling point: 3265 °C
2.8.4
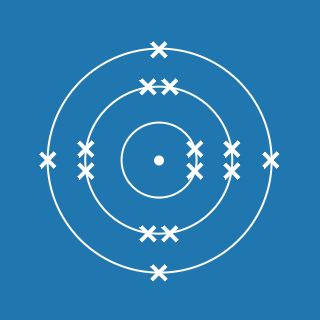
Phosphorus
Melting point: 44.2 °C
Boiling point: 280.5 °C
2.8.5
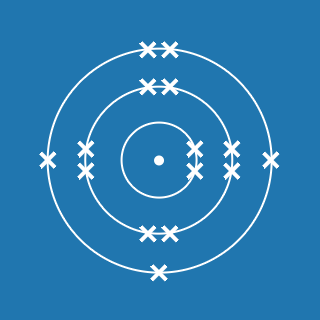
Sulfur
Melting point: 115.2 °C
Boiling point: 444.6 °C
2.8.6
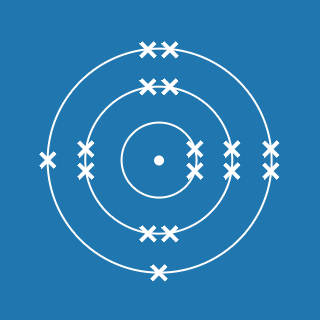
Chlorine
Melting point: –101.5 °C
Boiling point: –34.0 °C
2.8.7
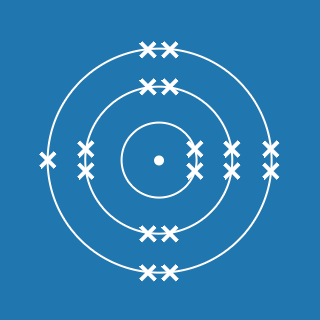
Argon
Melting point: –189.3 °C
Boiling point: –185.9 °C
2.8.8
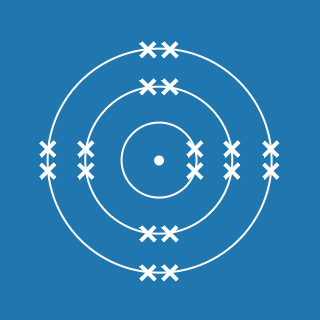
Potassium
Melting point: 63.5 °C
Boiling point: 759 °C
2.8.8.1
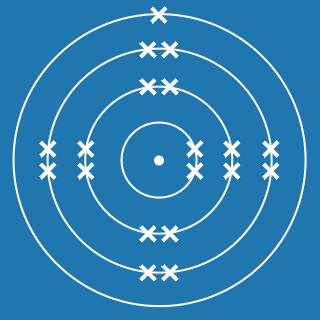
Calcium
Melting point: 842 °C
Boiling point: 1484 °C
2.8.8.2
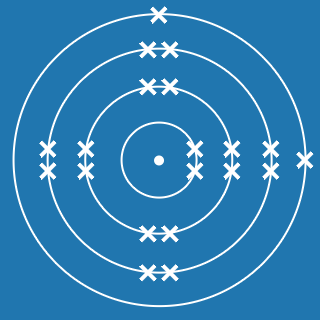
Scandium
Melting point: 1541 °C
Boiling point: 2836 °C
Titanium
Melting point: 1668 °C
Boiling point: 3287 °C

Vanadium
Melting point: 1910 °C
Boiling point: 3407 °C
Chromium ![]()
Melting point: 1907 °C
Boiling point: 2671 °C
Manganese
Melting point: 1246 °C
Boiling point: 2061 °C
Iron
Melting point: 1538 °C
Boiling point: 2862 °C

Cobalt
Melting point: 1495 °C
Boiling point: 2927 °C
Nickel
Melting point: 1455 °C
Boiling point: 2730 °C
Copper
Melting point: 1085 °C
Boiling point: 2562 °C
Zinc
Melting point: 419.5 °C
Boiling point: 907.0 °C

Gallium
Melting point: 29.76 °C
Boiling point: 2400 °C
Germanium
Melting point: 938.3 °C
Boiling point: 2833 °C
Arsenic ![]()
Sublimes at 615 °C
solid → gas
Selenium ![]()
Melting point: 221 °C
Boiling point: 685 °C
Bromine
Melting point: –7.2 °C
Boiling point: 58.8 °C
Krypton
Melting point: –157.4 °C
Boiling point: –153.4 °C
Rubidium
Melting point: 39.3 °C
Boiling point: 688 °C
Strontium ![]()
Melting point: 777 °C
Boiling point: 1377 °C
Yttrium
Melting point: 1526 °C
Boiling point: 2930 °C
Zirconium
Melting point: 1855 °C
Boiling point: 4377 °C
Niobium
Melting point: 2477 °C
Boiling point: 4744 °C
Molybdenum
Melting point: 2623 °C
Boiling point: 4639 °C
Technetium ![]()
Melting point: 2157 °C
Boiling point: 4265 °C
Ruthenium
Melting point: 2334 °C
Boiling point: 4150 °C
Rhodium ![]()
Melting point: 1964 °C
Boiling point: 3695 °C
Palladium
Melting point: 1555 °C
Boiling point: 2963 °C
Silver
Melting point: 961.8 °C
Boiling point: 2162 °C
Cadmium ![]()
Melting point: 321 °C
Boiling point: 767 °C
Indium
Melting point: 156.6 °C
Boiling point: 2072 °C
Tin
Melting point: 231.9 °C
Boiling point: 2602 °C
Antimony
Melting point: 630.6 °C
Boiling point: 1635 °C
Tellurium ![]()
Melting point: 449.5 °C
Boiling point: 988 °C
Iodine
Melting point: 113.7 °C
Boiling point: 184.3 °C
Xenon
Melting point: –111.7 °C
Boiling point: –108.1 °C
Caesium
Melting point: 28.5 °C
Boiling point: 671 °C
Barium ![]()
Melting point: 727 °C
Boiling point: 1845 °C
Hafnium
Melting point: 2233 °C
Boiling point: 4603 °C
Tantalum
Melting point: 3017 °C
Boiling point: 5458 °C
Tungsten
Melting point: 3422 °C
Boiling point: 5930 °C
Rhenium
Melting point: 3186 °C
Boiling point: 5630 °C
Osmium
Melting point: 3033 °C
Boiling point: 5012 °C
Iridium
Melting point: 2446 °C
Boiling point: 4130 °C
Platinum
Melting point: 1768 °C
Boiling point: 3825 °C
Gold
Melting point: 1064 °C
Boiling point: 2970 °C

Mercury ![]()
Melting point: –38.8 °C
Boiling point: 356.7 °C

Thallium ![]()
Melting point: 304 °C
Boiling point: 1473 °C
Lead ![]()
Melting point: 327.5 °C
Boiling point: 1749 °C
Bismuth
Melting point: 271.5 °C
Boiling point: 1564 °C
Polonium ![]()
Melting point: 254 °C
Boiling point: 962 °C
Astatine ![]()
Melting point: 302 °C
Boiling point: 337 °C
Radon ![]()
Melting point: –71 °C
Boiling point: –61.7 °C
Francium ![]()
Melting point: 27 °C
Boiling point: 677 °C
Radium ![]()
Melting point: 700 °C
Boiling point: 1737 °C
Rutherfordium ![]()
Mp (predicted): 2100 °C
Bp (predicted): 5500 °C
Dubdium ![]()
Melting point: unknown
Boiling point: unknown
Seaborgium ![]()
Melting point: unknown
Boiling point: unknown
Bohrium ![]()
Melting point: unknown
Boiling point: unknown
Hassium ![]()
Melting point: unknown
Boiling point: unknown
Meitnerium ![]()
Melting point: unknown
Boiling point: unknown
Darmstadtium ![]()
Melting point: unknown
Boiling point: unknown
Roentgenium ![]()
Melting point: unknown
Boiling point: unknown
Copernicium ![]()
Melting point: unknown
Boiling point: unknown
Nihonium ![]()
Melting point: unknown
Boiling point: unknown
Flerovium ![]()
Melting point: unknown
Boiling point: unknown
Moscovium ![]()
Melting point: unknown
Boiling point: unknown
Livermorium ![]()
Melting point: unknown
Boiling point: unknown
Tennessine ![]()
Melting point: unknown
Boiling point: unknown
Oganesson ![]()
Melting point: unknown
Boiling point: unknown
Alkali metals

Alkaline earth metals

Nitrogen group
(Pnictogens)

Oxygen group
(Chalcogens)

Halogens

Noble gases

Typical properties:
- high melting points
- high boiling points
- good electrical conductors
- good thermal conductors
- shiny when cut
- strong and malleable
Elements with properties in between
Typical properties:
- low melting points
- low boiling points
- poor electrical conductors
- poor thermal conductors
- dull
- brittle when solid
Physical state at room temperature:solid (s), liquid (l) or gas (g).
The weighted mean mass of the atoms
The number of protons in an atomic nucleus.
Also called the proton number.
Main differences between exam boards
| AQA | Edexcel | OCR | |
|---|---|---|---|
| Hydrogen, H | Immediately above Li | ||
| Relative atomic mass, Ar | Below chemical symbol All values to 2 decimal places, except bottom period and Tc (no value) |
||
| Atomic number | Above chemical symbol | ||
| Elements 57 – 71 | La shown, 58 – 71 called Lanthanides | ||
| Elements 89 – 103 | Ac shown, 90 – 103 called Actinides | ||
| Elements 112 – 118 | All shown | None shown | Cn, Fl, Lv only |