Melting and boiling points across period 3

Learning outcomes
After studying this page, you should be able to:
- describe and explain the trends in melting and boiling points across period 3.
Melting and boiling points
The table shows melting and boiling points for the elements sodium to argon.
| Element | Symbol | Atomic number | Melting point /K | Boiling point /K |
|---|---|---|---|---|
| Sodium | Na | 11 | 371 | 1156 |
| Magnesium | Mg | 12 | 922 | 1380 |
| Aluminium | Al | 13 | 933 | 2740 |
| Silicon | Si | 14 | 1683 | 2628 |
| Phosphorus | P | 15 | 317 | 553 |
| Sulfur | S | 16 | 392 | 718 |
| Chlorine | Cl | 17 | 172 | 238 |
| Argon | Ar | 18 | 84 | 87 |
The temperatures are given in kelvin, K.
You can easily convert K to °C and back again:
°C = K – 273 (e.g. 373 K = 100 °C)
K = °C + 273 (25 °C = 298 K)
Strictly speaking it should be 273.15 rather than 273, but the less precise value is acceptable at A Level.
Notice that there is no ° sign in front of the K symbol.
Description
The graph shows how melting points and boiling points vary across period 3.
When you click on the download symbol, you will be able to download the graph as an image file or pdf file, save its data, annotate it, and print it.
There is a lot going on in this graph, so it is often easier to divide it into three sections. The table below gives a brief summary of these sections.
| Elements | Na, Mg, Al | Si | P, S, Cl, Ar |
|---|---|---|---|
| Boiling point | Increases | Lower than Al | Lower than Si |
| Melting point | Increases | Higher than Al | Lower than Si |
| Forces overcome | Metallic bonding | Covalent bonds | van der Waals' |
When a substance melts, some of the attractive forces between particles are broken or loosened. The particles can move around each other but are still close together.
When a substance boils, most of the remaining attractive forces are broken. The particles can move freely and are far apart.
The stronger the attractive forces are, the more energy is needed to overcome them and the higher the melting or boiling point.
Explanation
Sodium, magnesium and aluminium
Sodium, magnesium and aluminium are all metals. They have metallic bonding, in which the nuclei of metal atoms are attracted to delocalised electrons.
Going from sodium to aluminium:
- the charge on the nuclei increases …
- the number of delocalised electrons increases …
- so the strength of the metallic bonding increases and …
- the melting points and boiling points increase.
Metallic bonding is often described as the attraction between positive metal ions and delocalised electrons. This is incorrect because metals still consist of atoms, but the outer electrons are delocalised and are free to move through the structure.
In a similar way, graphite (a non-metal) also has delocalised electrons. However, you don’t see the idea that it consists of carbon ions.
Silicon
Silicon is a metalloid with a giant covalent structure. Silicon has a very high melting point and boiling point because: all the silicon atoms are held together by strong covalent bonds … which need a very large amount of energy to be broken.
- the silicon atoms are attracted to each other by strong covalent bonds …
- which need a very large amount of energy so they can be broken.
The giant lattice structure of silicon is similar to that of diamond. Each silicon atom is covalently bonded to four other silicon atoms in a tetrahedral arrangement.
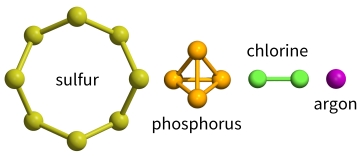
Phosphorus, sulfur, chlorine and argon
These elements are non-metals. Phosphorus, sulfur and chlorine exist as simple molecules with van der Waals’ forces between them. Argon is monatomic – it exists as separate atoms. There are are van der Waals’ forces between its atoms. The melting and boiling points of these elements are very low because:
- van der Waals’ forces are very weak forces of attraction …
- little energy is needed to overcome them.
Phosphorus exists as P4 molecules, sulfur exists as S8 molecules, chlorine exists as Cl2 molecules and argon exists as individual atoms. The strength of the van der Waals’ forces decreases as the size of the molecule decreases, so the melting points and boiling points decrease in the order:
S8 > P4 > Cl2 > Ar
The atoms in molecules of phosphorus, sulfur or chlorine are attracted to each other by covalent bonds. These bonds are much stronger than the van der Waals’ forces between the molecules: the covalent bonds do not break during the state changes of these elements.