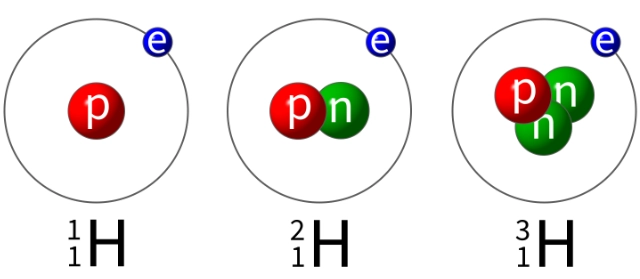
Atoms of an element that have the same number of protons but different numbers of neutrons. This means that their atomic number is the same but they have different mass numbers. Isotopes of a particular element have identical chemical properties because they have the same electronic configuration.
Go to the Glossary
